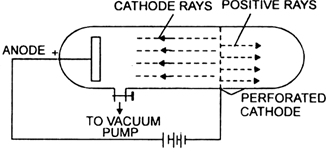
What are canal rays?
Canal rays, also known as anode rays, are streams of positively charged particles, specifically positive ions, that travel from the anode to the cathode in a discharge tube. They were discovered by Eugen Goldstein. These rays are the opposite of cathode rays, which consist of electrons.
Key characteristics of canal rays:
Positive Charge: They are made up of positively charged particles, which are ions that have lost electrons.
Origin: They are produced in gas-discharge tubes where high voltage is applied, ionizing the gas and creating positive ions.
Movement: The positive ions are attracted to the negatively charged cathode in the tube.
Mass: Canal ray particles have a significantly larger mass than electrons.
Discovery: They were first observed by Goldstein in 1886.