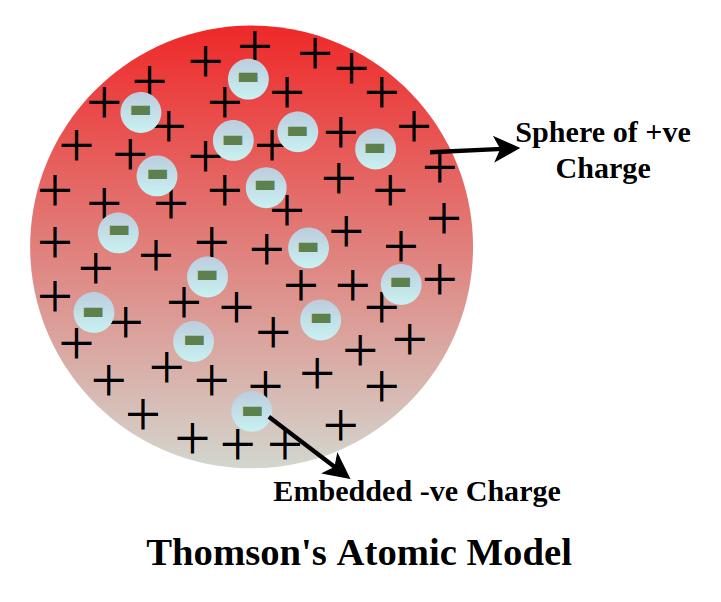
On the basis of Thomson’s model
of an atom, explain how the atom
is neutral as a whole.
In Thomson’s model, an atom is viewed as a positively charged sphere with negatively charged electrons embedded within it, like seeds in a watermelon. The total positive charge of the sphere is equal to the total negative charge of the embedded electrons, resulting in an overall neutral atom.
Here’s a more detailed explanation:
Positive Sphere:
Thomson’s model proposed that the atom was composed of a positively charged sphere of matter.
Embedded Electrons:
Negatively charged electrons were believed to be embedded within this positive sphere.
Equal Charges:
The key concept is that the magnitude of the positive charge in the sphere is exactly balanced by the magnitude of the negative charges of the embedded electrons.
Neutral Atom:
Because the positive and negative charges are equal and opposite, they cancel each other out, resulting in a net neutral charge for the atom as a whole.