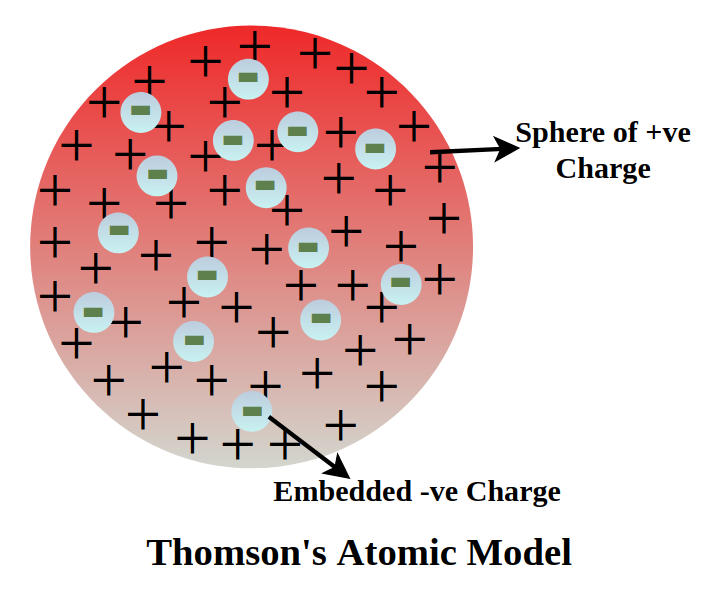
What are the limitations of J.J. Thomson’s model of the atom?
J.J. Thomson’s model, also known as the “plum pudding” model, had several key limitations. It failed to explain the stability of the atom, didn’t account for the presence of a nucleus, and couldn’t explain the results of Rutherford’s alpha-particle scattering experiment.
Here’s a more detailed look at the limitations:
Inability to explain atomic stability:
The model proposed that negatively charged electrons were embedded within a positively charged sphere, but it didn’t explain how these charges could remain together or how the atom could be stable.
Absence of a nucleus:
Thomson’s model did not include the concept of a central nucleus containing protons and neutrons, which was later established by Rutherford.
Failure to account for Rutherford’s experiment:
The model couldn’t explain why alpha particles, directed at a thin metal foil, would be deflected, rather than passing straight through, as predicted by Thomson’s model.
Lack of experimental evidence:
While Thomson’s model was based on the discovery of the electron, it lacked direct experimental support for its structure of the atom as a whole.