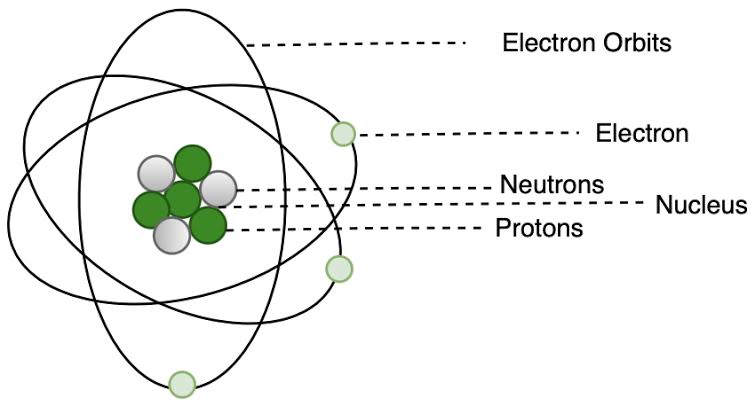
What are the limitations of Rutherford’s model of the atom?
Rutherford’s atomic model, while groundbreaking, had limitations. Specifically, it couldn’t explain the stability of the atom, nor did it account for the arrangement of electrons within the atom. Classical physics predicted that accelerating electrons would lose energy and spiral into the nucleus, making the atom unstable, which contradicted the observed stability of atoms. Additionally, the model didn’t explain why atoms emit light with specific wavelengths, which was a key feature of the hydrogen spectrum.
Here’s a more detailed look at the limitations:
Stability of the atom:
Rutherford’s model proposed that electrons orbited the nucleus. However, according to classical physics, these orbiting electrons would continuously emit electromagnetic radiation, lose energy, and eventually spiral into the nucleus, causing the atom to collapse. This contradicted the observed stability of atoms.
Electron arrangement and spectrum:
The model didn’t specify how electrons were arranged within the atom or why they didn’t fall into the nucleus. It also failed to explain the discrete line spectra of atoms, where specific wavelengths of light are emitted or absorbed.
Incomplete picture:
Rutherford’s model, while providing a better understanding of the atom’s structure than previous models, was incomplete and didn’t fully explain the behavior of electrons and the properties of atoms.