Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
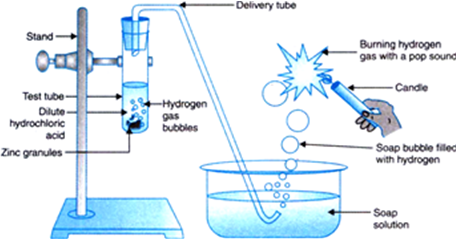
When an acid reacts with a metal, hydrogen gas is usually liberated. For example, when zinc metal reacts with dilute hydrochloric acid, hydrogen gas is released, along with zinc chloride. To test for the presence of hydrogen gas, you can bring a burning matchstick or candle near the gas. If hydrogen is present, it will burn with a “pop” sound due to the small explosion it creates.
Here’s a more detailed explanation:
Reaction:
When a metal reacts with an acid, a salt (a compound formed from the metal and the acid’s anion) and hydrogen gas are produced.
Example: Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g) (zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen gas).
Testing for hydrogen gas:
Hydrogen gas is easily identified because it is flammable and burns with a distinctive “pop” sound.
Procedure:
Collect the gas produced in the reaction in a test tube or gas collection bottle.
Bring a burning matchstick or candle to the mouth of the test tube or bottle.
Observe: If hydrogen is present, you will hear a “pop” sound as the gas burns quickly.
If you hear the pop sound, it confirms the presence of hydrogen gas.